Written by: Sarah Jacobs, BS, R.T.(R)(M)(CT), Breast Imaging Consultant
You’ve passed your yearly FDA/MQSA inspection and you think you can finally breathe easy. Then it happens - you get a notification from the American College of Radiology (ACR) that you are due for your facility’s accreditation renewal. You think “Ugh, didn’t I just go through this process?!”, and you start to panic… again. We tirelessly search for the perfect image and the perfect patient, only to find out that “perfect” is near impossible to find. The accreditation experience doesn’t have to be as stressful as we make it, though.
What does it mean to be ‘accredited’ and why is it so important?
Just as screening mammography continues to be the gold standard in terms of breast cancer detection, ACR Accreditation is also considered the gold standard in ensuring that patients receive the highest level of image quality and safety. The ACR is “comprised of more than 34,000 physicians and is the largest and oldest medical imaging accrediting body” (1). You can rest assured knowing that although the process of accreditation can be nerve-wracking, successful accreditation provides our patients with the highest quality imaging services available.
Going through the ACR accreditation process means that the facility has voluntarily gone through a rigorous inspection where four key areas are analyzed in order to confirm that the facility is adhering to the highest possible industry standards. These four areas are: Personnel Qualifications, Equipment Quality, Image Quality and Patient Protection (2).
Accreditation is a team effort.
In Mammography, poor image quality is the number one reason for failure according to the ACR. In 2015, the American College of Radiology (ACR), found that of all clinical images which were deficient on the first attempt at accreditation, 92% were deficient in positioning (3).
The ACR accreditation process can be traumatic for both brand new and experienced mammographers alike. As mammography technologists, we tend to take on much of the responsibility of accreditation ourselves. This also means that we may put the deficiency or failure on our shoulders, as well. Although the technologist performs the mammogram, the responsibility of proper positioning is shared by technologists and interpreting physicians (3).
It’s important to remember that we are a team and accreditation is the responsibility of every technologist and breast imaging radiologist at our facility.

The Key to Accreditation: Preparation
First and foremost, accreditation preparation is key. Take the time to review the ACR’s website on Mammography Accreditation. Ensure that all required documentation is updated and current. Ms. Louise Miller wrote an article in recently that outlines 5 Positioning (and Other) Tips To Help You Pass ACR Accreditation (4).
You’ve meticulously prepared, but accreditation deficiency or failure occurs.
What happens when you’ve done everything you can to adequately prepare, but your facility still fails the accreditation process? Take a deep breath and don’t stress. It’s important to understand the reason for failure. The final report issued to the Lead Interpreting Physician will give specific reasons for accreditation failure as well as next steps for corrective action.
The most common reasons for failure/deficiency (5):

Understand your options depending on if this is the unit’s 1st, 2nd, or 3rd attempt at accreditation. The table below outlines a facility’s options:
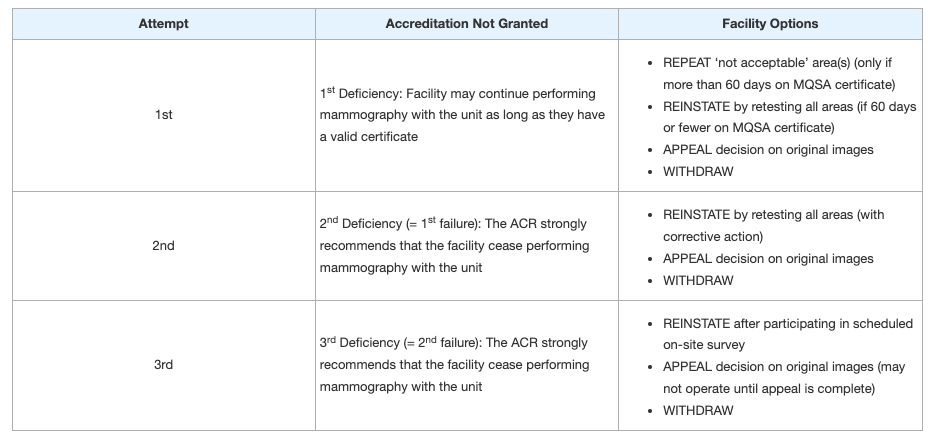
Table courtesy: ACR (6)
Understand the Key Terms
REPEAT:
The facility may REPEAT any “not acceptable” areas; however, there must be more than 60 days left on the MQSA certificate date. If the unit is eligible for a repeat submission, it will automatically be placed in the repeat cycle. If you are REPEATING a test for a deficiency, you should only submit images from the deficient test (i.e., all clinical images, both fatty and dense, and/or the phantom). Repeat exams are new exams not previously submitted for ACR review and should be collected after the date on the deficiency report. This allows reviewers to assess the overall improvement in image quality since the previous deficiency and to determine whether the facility took all reviewer comments into consideration (6).
REINSTATE:
If there are 60 days or less on the current MQSA certificate, the facility may choose to REINSTATE accreditation by re-testing all areas of the application. This includes the full accreditation application, all testing materials and a reinstatement fee.
APPEAL:
If the facility and leadership disagree with the ACR’s decision of deficiency or failure, they may APPEAL the decision on the original images sent in for accreditation within 30 days of the final report. The Lead Interpreting Physician (if clinical images are being appealed), or Medical Physicist (if phantom images are being appealed) must submit a letter detailing the reason for the appeal. Images will be forwarded to an arbitrator with a copy of the previous review and the appeal letter written by the facility. If the facility would like to appeal, they must contact the ACR for further instructions.
WITHDRAWAL:
A failed unit may be withdrawn if the site no longer performs mammography on that unit. If the facility would like to withdraw the unit, they must contact the ACR for instructions.
Once your facility determines their next steps based on the table above and the options available to them, the deficiency or failure isn’t quite so daunting. An important aspect to remember is that the ACR is available to help in these challenging circumstances. Their call center can help answer any questions you or your facility have and guide you in the application process. Support specialists are available 8:30am-5:00pm EST Monday through Friday at 1-800-227-6440.
ACR In the News:
Many of you are aware that currently, when submitting for DBT accreditation, facilities have to submit two separate applications: one for Full-Field Digital and one for DBT. But here’s some great news! The ACR Accreditation team will soon unveil an updated accreditation database called ACRedit Plus (7). The new user-friendly interface boasts enhanced features and one of the main features for facilities with digital breast tomosynthesis (DBT) units will no longer have to apply as two separate units for Full-Field Digital and DBT. Instead, these units will apply as a single unit with modules. Facilities under this scenario will see an FDA-approved fee decrease over the current model as well as benefit from the more streamlined process.
It’s important to remember that going through ACR accreditation is a process that should be shared by your team and not a task that’s resting on one individual’s shoulders.
If a failure or deficiency occurs, don’t panic! Stay calm and use the table and resources above to determine your facility’s next steps. When challenging questions arise, call the ACR for directions or additional support. Getting that final letter from the ACR stating that your unit has passed accreditation is so much sweeter after going through a challenging circumstance such as a deficiency or failure. Stay positive and look for the light at the end of the tunnel.
References:
- https://aboutimi.com/acr-accreditation-what-it-means-and-why-it-matters/
- https://www.acraccreditation.org/modalities/mammography
- https://www.fda.gov/radiation-emitting-products/mqsa-insights/poor-positioning-responsible-most-clinical-image-deficiencies-failures
- https://mammographyeducation.com/positioning-tips-acr-accredidation/
- https://acrsupport.acr.org/support/solutions/articles/11000047152-frequent-deficiencies-revised-2-2-2020-
- https://acrsupport.acr.org/support/solutions/articles/11000047157-options-following-a-failure-or-deficiency-mammography-revised-12-12-19-
- https://www.acraccreditation.org/Coming-Soon



